There are two issues that should be considered when preparing a sample for electron microprobe analyses. |
| Importance of Flat, Polished Surfaces |
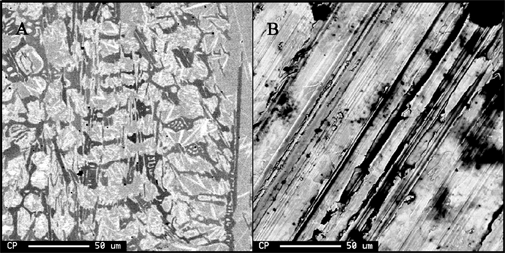
To the right are backscattered electron images of two surfaces of the same stainless steel sample. The surface on the far right (B) was cut with a diamond saw, but not polished. The other surface (A) was polished down to the one micron level. One can clearly see detailed structures in the image of surface A, however these are completely masked by the roughness of surface B. In the diagrams below, one can see why rough or tilted surfaces will cause problems during the analyses. |
 |
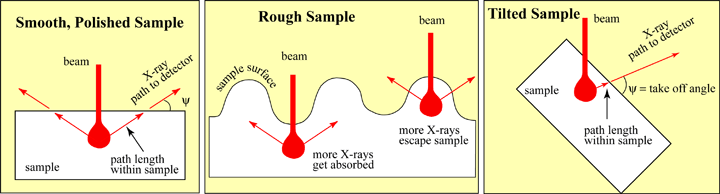
| With a polished surface, the counts are controlled by the abundance of the elements. The path length that the X-rays follow through the sample are constant, and therefore the percentage of X-rays that gets absorbed by the sample is constant. However, with a rough surface the path length varies, and therefore the amount of absorption varies. More X-rays will escape from the sample when the beam is hitting a ridge, than when the beam is hitting within a valley. Therefore the counts will be higher from the ridges, even if the composition is identical. A tilted sample will cause the same type of problem. By tilting the sample, the X-rays path length is shorter, therefore there will be less absorption and a higher X-ray count rate. Since the X-rays' counts on an unknown are compared to the counts on a standard, the variability makes it impossible to get good quantitative elemental analyses. |
| Conductive Coatings |
In order to get good analyses the surface of the sample must be conductive. If the sample itself is not conductive, then a conductive coating must be deposited onto the surface. If not, a charge will build-up on the surface and begin to deflect the beam. If the sole purpose is to image the sample, then many metals such as Au, Pt, or Pd, will work as a sputter coating. However, these high atomic number metals also absorb low energy X-rays being emitted from the sample. For this reason, carbon coating is used almost exclusively for microprobe analyses. Carbon, being a low atomic number element, will absorb fewer of the low-energy X-rays. There are exceptions and some analysts will experiment with aluminum coatings when carbon is one of the elements being analyzed for. The thickness of the carbon coating is also important, and great care should be taken to ensure that the same thickness of carbon is deposited on the unknown sample as was deposited on the standards. For light element analyses, it is usually suggested that the standards and unknowns be coated at the same time to ensure that the coatings are the same thickness (see Oxygen Analysis). |