| McSwiggen & Associates |
| /Tech Notes/Carbon |

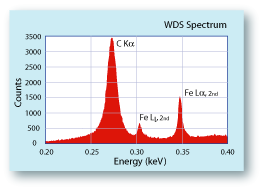
| Carbon (C, Z=6) is an important component of many
materials, including steels, carbides, and carbonate
minerals. However, carbon can be difficult to analyze
in low concentrations, because of the pervasiveness of hydrocarbons in most vacuum systems, and because many samples have been carbon coated to make the surface conductive. In order to measure small concentrations of carbon in a sample, carbon coating must be avoided. For conductive samples this is not a problem; however for non-conductive samples, alternative methods must be explored. Some analysts use very thin coatings of sputtered aluminum. Aluminum has a relatively low atomic number, so it absorbs fewer of the low energy carbon X-rays than more typical metal coatings like gold or platinum. Another factor to consider is the system carbon. This is carbon that has become attached to the surface of the sample and hydrocarbons in the vacuum of the electron column. Hydrocarbons in the vacuum can be minimized by maintaining a clean system and by using cold traps. A liquid nitrogen baffle above the diffusion pump can trap diffusion oil before it gets into the system. A liquid nitrogen "cold finger", a couple of millimeters above the sample surface, can trap the hydrocarbons before they migrate into the electron beam. Such a device can reduce the rate of carbon build-up on the surface by an order of magnitude.
|
 |
|
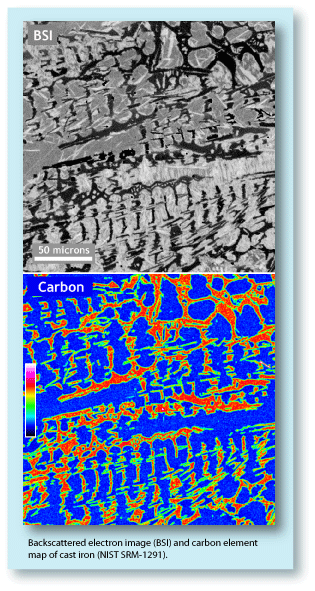
Surface contamination can be minimized by cleaning the surface of the sample. Care should be used, though, if acetone or alcohol is applied to the surface. Both have been shown to dissolve and smear epoxy mounts across the surface of a sample. Just water and a soft cloth work very well for cleaning sample surfaces. If more aggressive cleaning is required, then repolishing the surface should be undertaken with a very fine polish. The effects of surface contamination can also be minimized by using a higher acceleration voltage, which produces a greater depth penetration. Therefore the percentage of the interaction volume that will be contained in the surface film will be much smaller and, as a result, the film will have less of an effect on the analyses. The downside of using a higher accelerating voltage is that the interaction volume will be larger and therefore the analysis will be sampling a larger area. Another strategy for minimizing the effects of surface contamination and hydrocarbons in the vacuum is to use a calibration curve for the analyses. The advantage of a calibration curve is that the contamination problems mentioned above will be the same for both the standards and unknowns, and therefore will be eliminated. However, calibration curves can only be used when the standards and unknowns have a similar composition, and therefore the matrix effects will be the same. |
 |
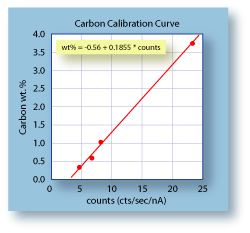 |
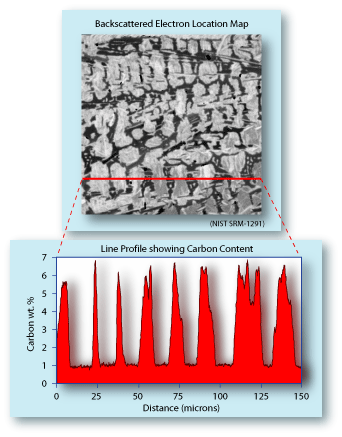
An example of a calibration curve is shown to the left. It was
developed for measuring carbon in steel, and should only be
used for samples that have a matrix that is predominantly iron. The
standards used to determine the calibration curve were all
NIST material (SRM-C2423, -1764, -1762, -1761).
The plot shows that a steel sample with no carbon in it still produces about 3 counts/sec/nA. This represents the combination of the background X-ray counts plus the system carbon counts. Using such a calibration curve it is possible to determine the carbon content of steel as low as a tenth of a weight percent. |